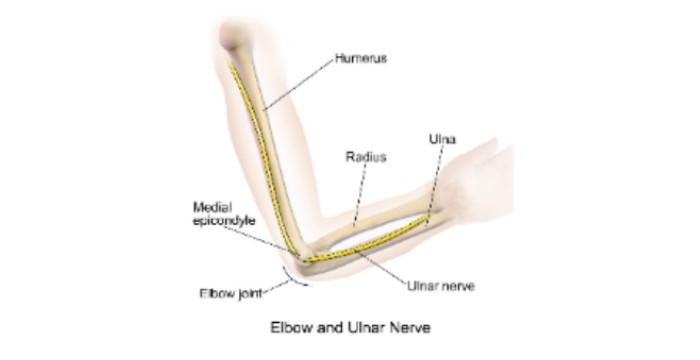
Cubital Tunnel syndrome (also known as CuTS, and Ulnar Nerve Compression) is a common type of nerve problem in the arm resulting in pins and needles, pain and /or weakness in the hand. It happens when the ulnar nerve, one of the three main nerves in your arm become squashed (compressed) or irritated. The condition affects approximately 6% of the population. For those whose symptoms are not managed, doctors can offer surgery. This surgery is called ulnar nerve decompression surgery.
There are currently two ways of performing ulnar nerve decompression in the UK, they are called open decompression surgery and endoscopic decompression surgery.
Both surgical techniques have potential advantages and disadvantages and the UNDER study is investigating which is better both in the short and longer term for patients with CuTS, and the results of this study will provide definitive evidence to guide future decision making for patients and clinicians in the treatment of CuTS.
This study will consist of two phases. The first to assess the training of experienced decompression surgeons in the endoscopic technique and how well patients recover post-surgery as, although both techniques are performed within the NHS and the surgeon has been trained in performing both techniques, endoscopic decompression is less widely used. The second phase will be a randomised control trial where the two techniques will be compared.